usp class vi elastomers
However some applica-tions such as implantable devices are extremely complicated. Meeting both FDA and USP criteria guarantees that the elastomer is acceptable for sanitary process applications and the elastomers or extracts from the elastomers will not be harmful to human health.

Usp Class Vi Standard Anderson Negele North America
Please contact the division for assistance in selecting materials in these situations.
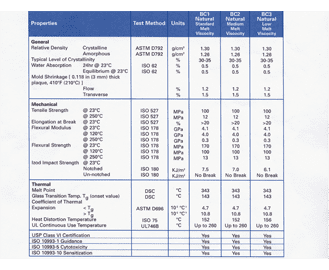
. Designates baseline requirements 5. Table 1 shows our standard programme FDA compliant com-pounds which can be produced in a few days. Ethylene Propylene is a elastomer that demonstrates great weather aging and ozone resistance excellent water and chemical resistance great gas permeability and aging due to steam exposure.
The Premium Package includes all elastomers in the wetted path of the SLA Series Biotech including O-rings and Valve Seats are USP Class VI and ADI Free and include a full package of certificates. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for Healthcare Products Specify JBL USP Class VI and FDA Silicone Materials Technical Bulletin The Parker JBL Division offers a wide range of USP Class VI and FDA elastomeric materials for the Healthcare Industry. USP Class VI elastomers.
Sil 714001 USP class VI Silicone 1 70 Yes transl. Most applications are fairly benign to elastomers. Most applications are fairly benign to elastomers.
It is used in the assembly of medical devices and is capable of withstanding repeated sterilizations including radiation ethylene oxide chemical sterilants and especially autoclaving. EPDM however ranks poorly when exposed to oil and solvents but demonstrates average. Based on the environment different elastomers can be recommended.
Ecdel 9966 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance needed in a variety of flexible packaging including flexible medical and pharmaceutical packaging applications. FAR 25853a for flame retardancy. Provides a high-level introduction to elastomer chemistry manufacturing technology and the post processing of components 3.
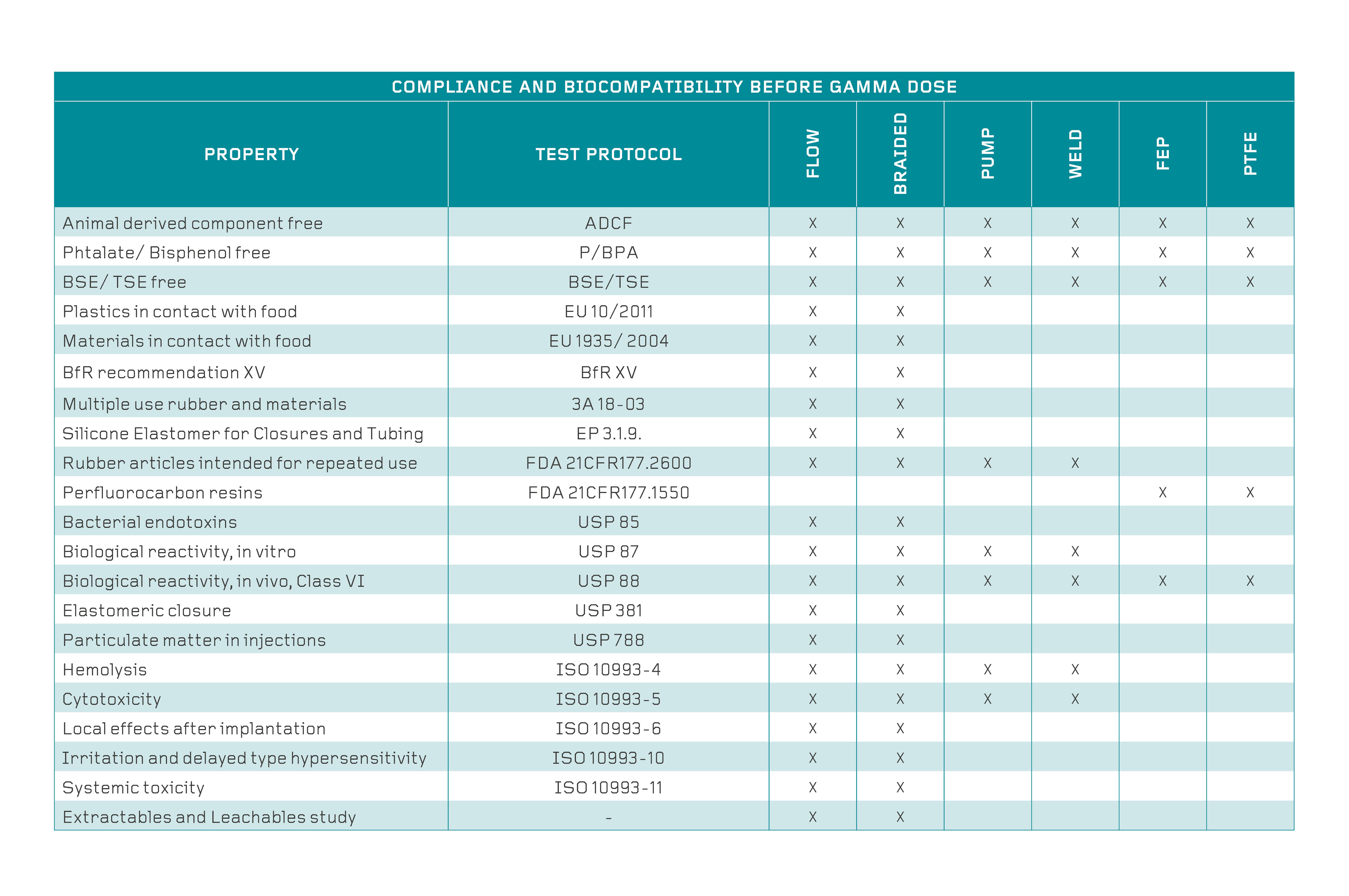
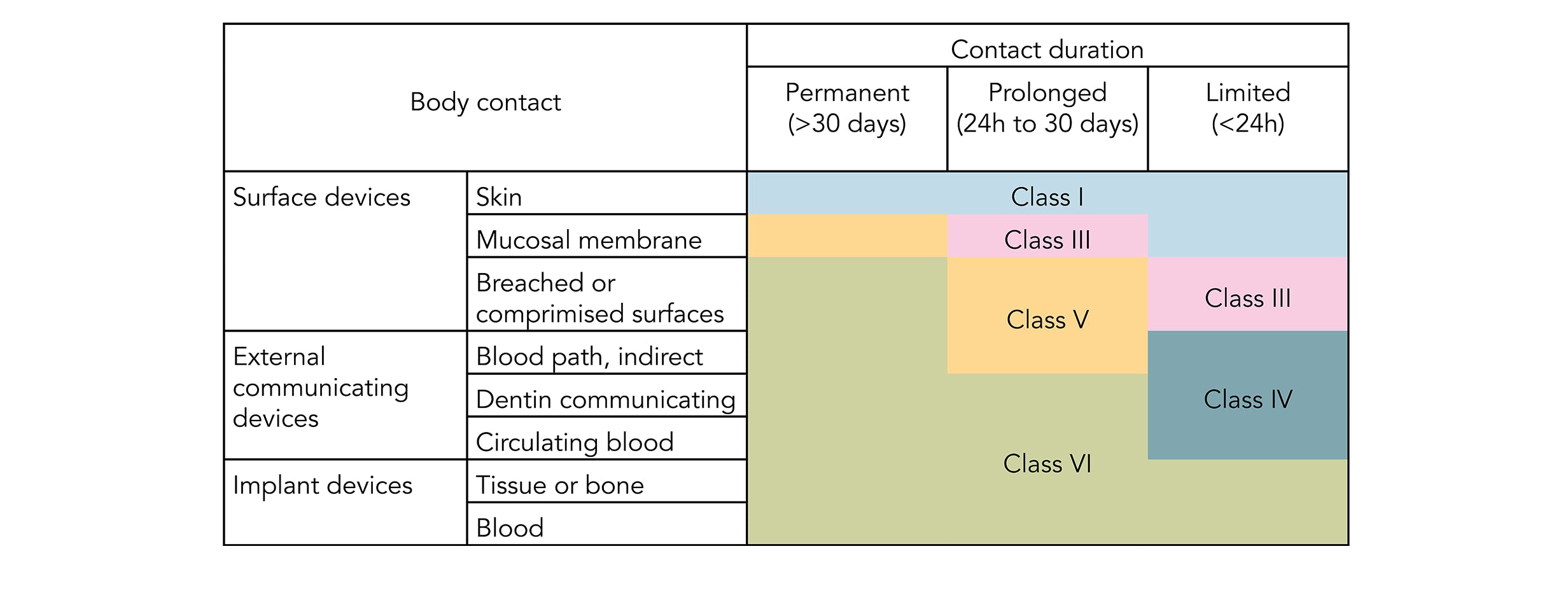
For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. However some applica-tions such as implantable devices are extremely complicated. PPE manufactures medical and pharmaceutical grade gaskets and seals including O-rings sanitary gaskets and other high performance seals from a range of 15 USP Class VI compliant elastomers-.
UL 94V-0. Please contact the division for assistance in selecting materials in these situations. FDA CFR 175300.
Available as USP Class VI and are therefore ideally suited for medical product applications. MIL-STD-810G for fungus resistance. Ia USP Class VI andor ISO 109933 will be required.
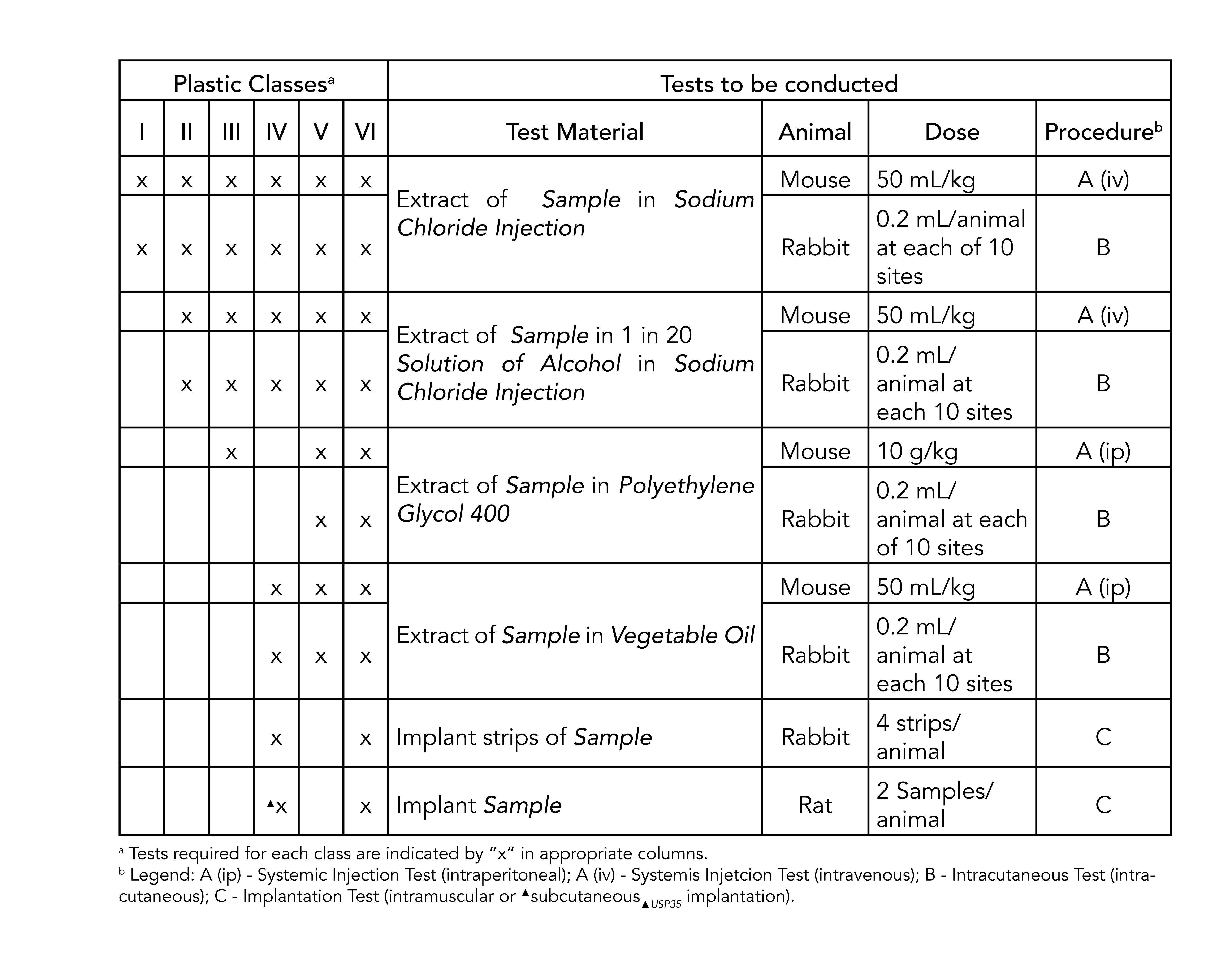
These tests correspond to numbered classes and use. United States Pharmacopeia USP 26 NF21 2003 Class VI Three chapters are applicable to elastomers plastics and polymeric materials. Class VI elastomers are highly sought after by those in the medical and pharmaceutical industries.
Discusses identification testing A workshop Modernization of USP Packaging Standards for Glass and. Sil 714002 USP class VI Silicone 1 70 Yes transl. Ia USP Class VI andor ISO 109933 will be required.
Ideal for process systems requiring intensive CIP and SIP regimes or aggressive process media. Universal material used in a wide variety of applications that require outstanding performance. 7 USP Class VI materials.
Additionally 22 Material Certifications certifying the composition of all materials in the wetted path and International Calibration Traceability calibrations traceable to NIST or. The TPE product series were designed for injection molding and extrusion applications to impart a soft touch a rubbery feel to end. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment.
FDA Compliant and USP Class VI Compliant Elastomers. 7 USP Class VI materials. ISO 10993-5 for cytotoxicity.
Ethylene-Propylene EP EPT EPDM. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. This is because they have a wide range of uses and are the safest types of seals and components for all types of medical equipment.
Class VI Section Biological Reactivity Test in Vivo. Explains basic functional characteristics of components 4. MIL-STD-883J for thermal stability.
Materials compliant to FDA 21 CFR2400 d 3-A USP Class VI Cytotoxicity USP 87 Typical Healthcare Medical Applications. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. The USP Class VI approved elastomers are.
Most applications are fairly benign to elastomers. Silicone S0317-60 A red colored material that offers the widest temperature range of all the material choices - going from -65F to 400F. USP Class VI for biocompatibility.
Ecdel 9966 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance needed in a variety of flexible packaging including flexible medical and pharmaceutical packaging applications. UL 1203 for explosion-proof. These materials can be supplied as precision extruded and.
These products range in hardness from Shore A 13 to 84. UV10TKMed is USP Class VI approved and meets ISO10993-5 for cytotoxicity requirements. Providing FDA conformance with new or replacement com-.
This material is good in dry heat and has good ozone resistance.

Valley Seal Introducing Usp Fda Specialty Elastomers
Meaning Of Usp Class Vi Standard United Kingdom

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
![]()
Usp Vi Silicones Fda Approved Pronat

Usp Class Vi Seals Compliant To Food Grade Standards Barnwell

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Parker Medical Fda O Rings Sealing Devices

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California

Newman Sanitary Gasket Fda And Usp Class Vi Quality Viton Gasket Manufacturer